Nov 23 2015
Applause for my mitochondria
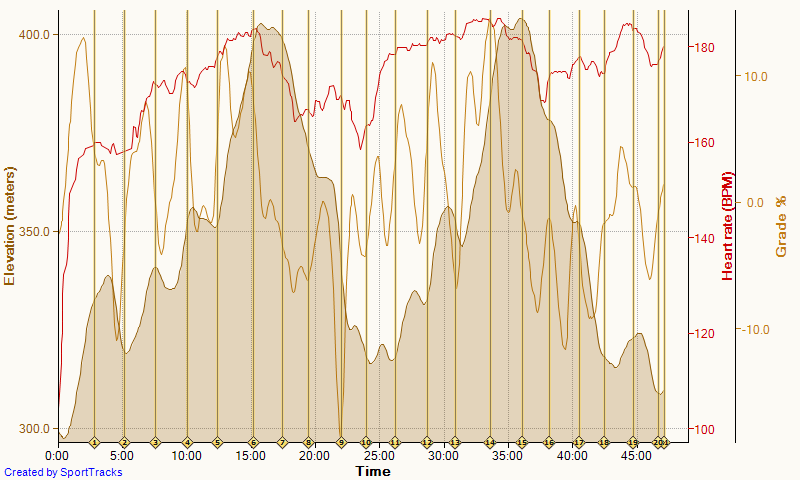
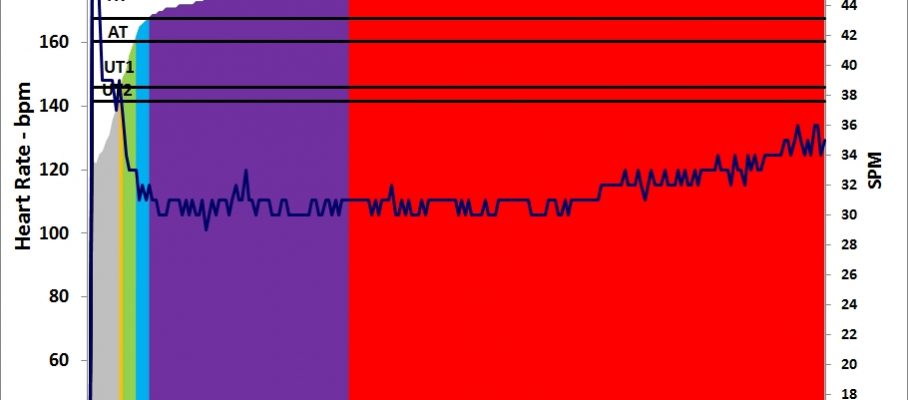
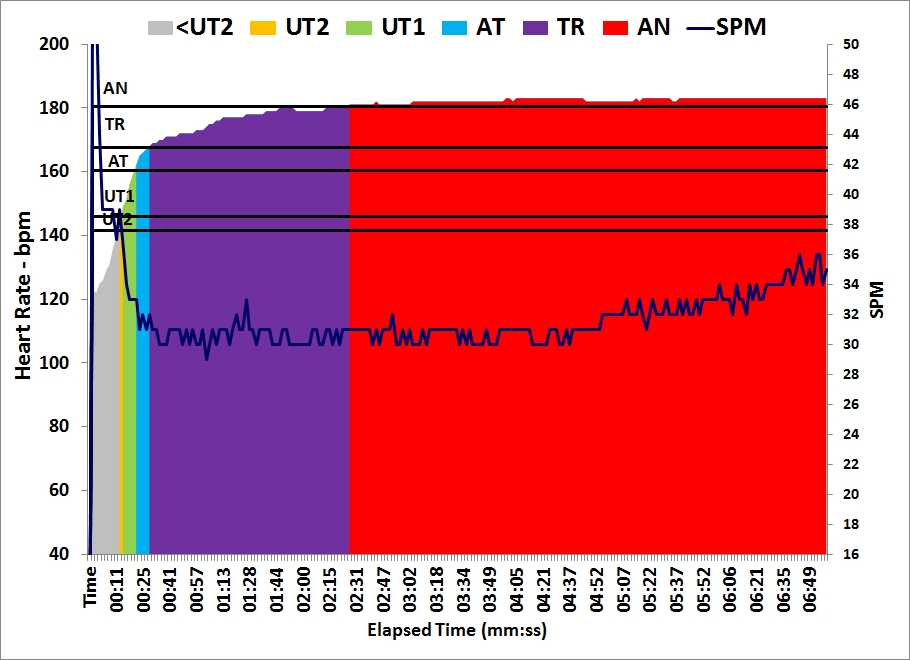
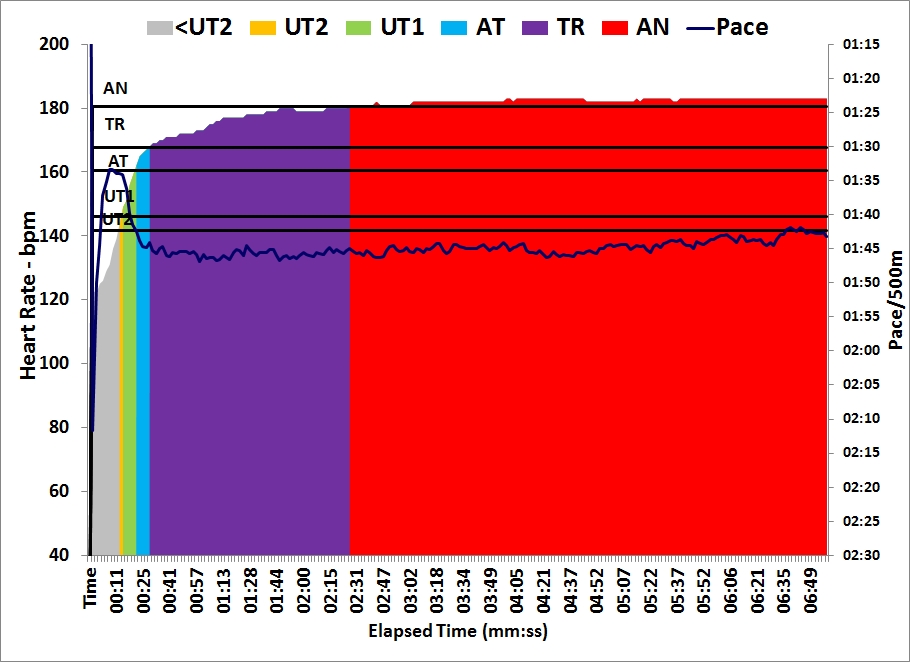
The plan asked for 5x1500m intensive session but I decided to swap sessions and do another lactate test. After yesterday’s test, I decided that the next test would be to measure lactate after a steady state session at 200W. I slightly changed that to a 6x10min/2R to keep the protocol as close as possible to yesterday’s.
Temperature in my rowing cellar was 8 degrees C. Nice, because this is also the place where I stock and cool my liquid carbs (read: beer).
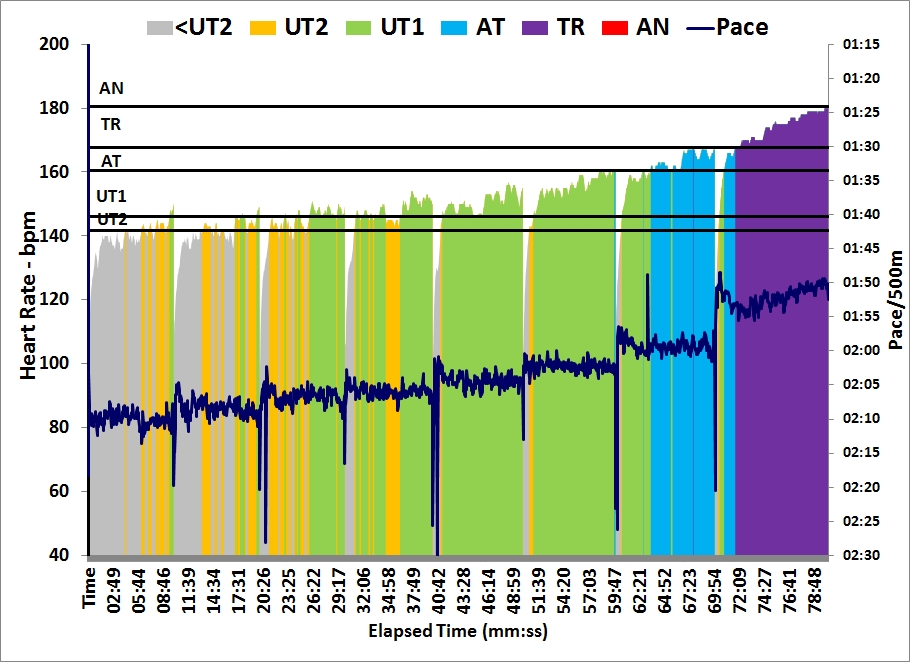
Here are the data:
Workout Summary - Nov 23, 2015
--|Total|-Total-|--Avg--|-Avg-|Avg-|-Avg-|-Avg
--|Dist-|-Time--|-Pace--|Watts|SPM-|-HR--|-DPS
--|14973|60:00.0|02:00.2|201.5|23.0|156.4|10.9
Workout Details
#-|EDist|-Etime-|-SPace-|Watts|SPM-|AvgHR|DPS-|Comments
01|02493|10:00.0|02:00.3|200.9|22.1|151.9|11.3|Lo
02|04985|20:00.0|02:00.4|200.6|22.7|154.2|11.0|0.9 mmol/L
03|07481|30:00.0|02:00.2|201.5|22.9|157.0|10.9|0.9 mmol/L
04|09975|40:00.0|02:00.3|201.1|23.3|157.8|10.7|Lo / 0.7 mmol/L
05|12474|50:00.0|02:00.0|202.4|23.5|158.0|10.6|1.1 mmol/L
06|14973|60:00.0|02:00.0|202.4|23.3|159.5|10.7|1.0 mmol/L

Again the first measurement was a failure because of a bad stab, not enough blood. This lactate testing is also a test of self-control. Of course I rowed the second interval angry and disappointed. After that interval I reverted to “multiple stabbing”, i.e. stabbing at 2 or 3 spots on three fingers, to see if I could discover a good spot.
All good spots were on my little finger. Some milking was needed to get good drops.
Also, I watched in frustration how, during the row, the leaks started to produce blood. I took my right hand off the handle during the recovery and watched impressive blood drops forming during the first three minutes of the interval. Interestingly, also from “old” stab holes. Then, when it was time to measure, I would wipe and wash and wipe dry, then stab, and then it would take a long time for a usable droplet to develop. The cleaning and stabbing took about 20, 25 seconds. Waiting for the blood another 20 seconds.
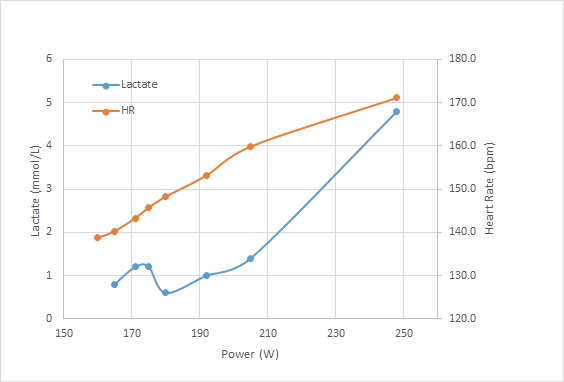
Here is the bloody graph:
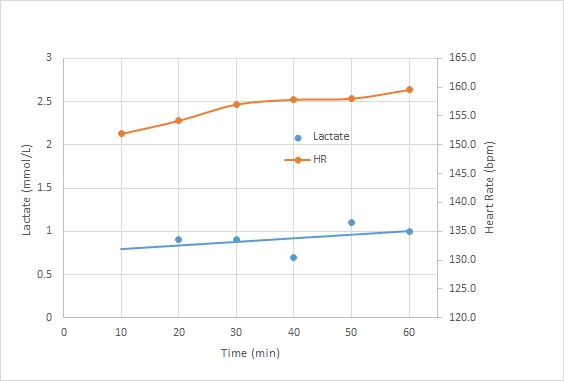
A couple of points. I am still suspicious of the results, but:
- It is possible that the long time between measurement and exercise causes the measurement to be off. But then, if I would be above the threshold, the lactate flushing from the muscles would cause blood lactate to increase initially. Measuring numbers well below 2.0 indicates I am really well in the aerobic training band.
- The 40 minute date point was a second strip, measured a bit later than usual, because I ruined the measurement on the first strip by accidentally switching off the meter during the measurement. So even if this data point is lower because it was measured later, then that would indicate that I am extremely good at flushing lactate …
- It could be that by taking relatively long breaks I “reset” my lactate levels and I was basically doing 6 separate 10 minute intervals, instead of one 60 minute continuous row. That may be true, but that would also point to fast flushing of lactate during the breaks.
My current theory is that I have very happy mitochondria, busily oxidizing lactate and running their Krebs Cycle at maximum speed. That would also explain unusually low steady state lactate levels and a lactate threshold which seems to be at a higher percentage of 2k speed than my other fellow blogging rowers.
It is true that even in periods where I trained less frequently, before I took up rowing again, I have been a happy long distance runner. Not ultra long distance, but just churning out the 10k and 12k runs over a weekend, or even on the treadmill.
Still reluctant to say that I “own” the 200W as a steady state training intensity.
I will give the lactate tester a few days off. Tomorrow is the 5x1500m session, and then on Thursday or Saturday I will do a continuous 60 minute row at 200W and measure lactate afterwards.









Nov 24 2015
5x1500m
Ben Redman posted a link to an interesting series of articles on endurance training in his latest blog post. Great reading to stimulate thoughts on rowing training for time pressed people.
My ride to work this morning was a bit slower than usual, because it was mighty cold and the mist was freezing to some sections of the road. I didn’t fancy a fall so I drove carefully.
Riding my bike I got cold hands, which made me think about my super-low lactate readings of the past two days. I guess my difficulty to get good blood flowing is to do with the low temperature in my rowing basement. The thermometer on the wall indicates something between 8 and 12 degrees C.
I also expressed fear that the low temperature might cause the readings to be lower than normal.
I posted these thoughts on the Lactate training thread on the Free Spirits rowing forum. By the end of the day Boris (“dr3do”) had responded that indeed according to him lower temperatures give lower readings and I should do my measurements at normal room temperature.
Then I made the mistake of posting a response before thinking, and as any new convert going through excitement, expectation, disappointment and frustration, I overreacted. On a public Forum. After 20 years of being on the internet I should know better.
Anyway, I apologized and I hope everything will calm down and be back to normal.
This low temperature thing is a bummer. I love to train in my chilly basement. To get the facts, I moved the thermometer to the basement floor where I prepare my lactate strips:
Indeed, lower than the 8 degrees measured on the wall.
On Thursday I will do an experiment to confirm (or, hopefully, falsify) my fear. I will take two strips to the basement and leave one prepared in a heated room. I will do a 60 minutes steady state, and take a lactate measurement immediately after. Then I will do a 1km very light cooling down and take another measurement. Finally, I will walk up the stairs and take a measurement with a warm strip. The two last measurements should be close. If the “warm” measurement gives a higher reading than the second “cold” measurement, I am in trouble.
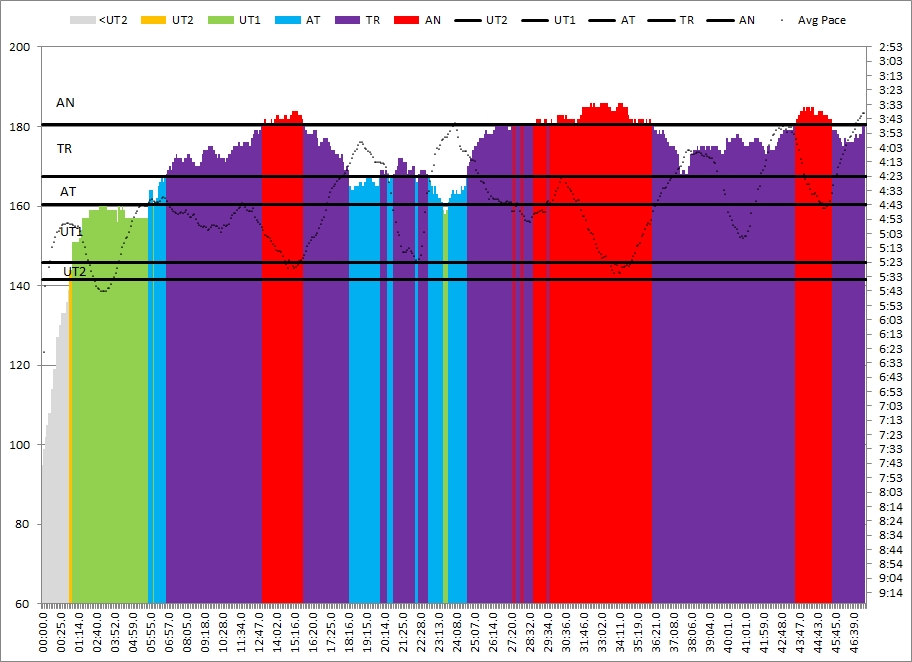
Today’s training was a good old 5x1500m. Target pace was 1:50.7 which I was not looking forward to. At least I was able to channel my forum faux-pas frustration and focus on the splits.
Workout Summary - Nov 24, 2015
--|Total|-Total-|--Avg--|-Avg-|Avg-|-Avg-|-Avg
--|Dist-|-Time--|-Pace--|Watts|SPM-|-HR--|-DPS
--|07500|27:31.4|01:50.1|262.3|27.8|165.9|09.8
Workout Details
#-|EDist|-Etime-|-SPace-|Watts|SPM-|AvgHR|DPS-|Comments
01|01500|05:30.2|01:50.1|262.5|27.1|158.2|10.1|
02|03000|11:01.7|01:50.5|259.4|27.5|165.0|09.9|
03|04500|16:33.3|01:50.5|259.2|27.3|167.9|09.9|
04|06000|22:04.8|01:50.5|259.3|27.9|167.8|09.7|
05|07500|27:31.4|01:48.9|271.2|29.2|170.3|09.4|
Here’s a picture of my erg room wall with the scores on the doors:
The scribbled equations were written during the breaks of the 5x1500m. I was thinking about enzymes and the temperature dependence of their activity. Being a physicist I couldn’t come up with anything better than something looking like an Arrhenius equation. That doesn’t bring me any further because I don’t have a clue what typical activation energies would be for enzymes, let alone if they follow Arrhenius equation type behaviour. Also I would have to guess about the temperature in the strip. The plastic housing looks quite a good heath insulator. Then you pour warm blood into something that is probably around 5 degrees C. We are looking at a temperature difference that is probably a bit smaller than 294K – 278K. Basically if the reaction rate would half between these two temperatures, then the activation energy would be 31 kJ/mol. However I am afraid that with enzymes as a catalyst, things are probably not so simple.
May look into it but I fear the experiment is a more useful way to determine the effect.
Tomorrow is a resting day. An old rowing friend has a business trip to Prague, so I will join him for a beer and a dinner. Here is the two of us rowing the double in Amsterdam in 1993. I am the stroke rower and Ele Jan is the bow.
By sanderroosendaal • Uncategorized • 11 • Tags: 5x1500m, concept2, erg, OTE, rowing, training